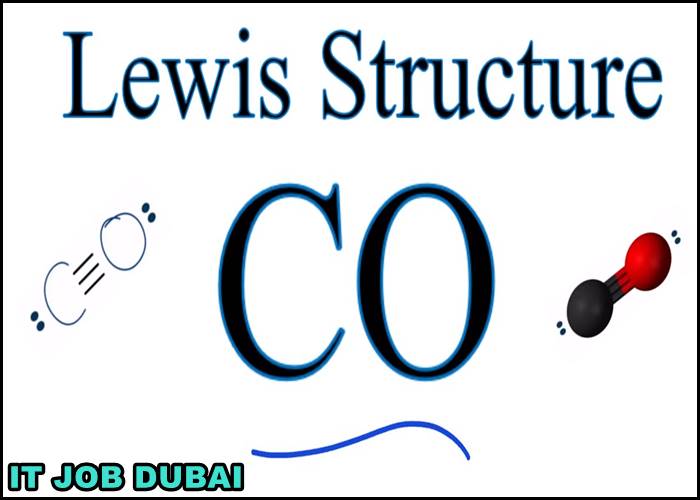
What is the Lewis Structure of CO?
Lewis structures, also known as Lewis point formulas, Lewis point structures, electronic point structures, or Lewis electronic point structures (LED), are diagrams that show the bond between atoms in a molecule, as well as the isolated electron pairs that may exist. in the molecule.
A Lewis structure can be drawn for any covalently linked molecule, as well as for coordinating compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article The Atom and the Molecule.
Lewis structures extend the concept of the electron point diagram by adding lines between atoms to represent shared pairs in a chemical bond.
What is the Lewis Structure of CO?
Well, I like to invoke charge separation in this instance… We got carbon, Z=6Z=6, and oxygen, Z=8Z=8… There are thus 14 electrons … FOUR of which formally constitute the 1s21s2 SET on each atom, which we do not conceive to be involved in bonding…
And so for the carbon monoxide MOLECULE, we distribute 10 electrons, or 5 pairs of electrons…
And thus −:C≡O:+−:C≡O:+….5 electron pairs distributed as required (the alternative designation is:C=O..::C=O..:). Most inorganic chemists would use the former representation, given that when carbon monoxide binds as a ligand to transition metal centers (and it has quite an extensive coordination chemistry!), it ROUTINELY binds thru the carbon, NOT the oxygen…the C−OC−O bond length of 1.128×10−10∙m1.128×10−10•m is also consistent with a triple bond…
Why does CO form a coordinate bond?
Carbon has atomic number 6 which is its electronic configuration is 1s2 2s2 2p2. Oxygen has an electron configuration of 8 which its atomic number is 1s2 2s2 2p4.
In a normal molecule, carbon and oxygen form a double bond.
That is to say, carbon contributes its 2 electrons and oxygen contributes its two electrons to form a double bond. Now the carbon is left with 2 valence electrons and it has a total of six electrons and the oxygen has filled its byte.
Carbon is therefore relatively unstable because, according to the byte rule, atoms combine to achieve the rare gas configuration closest to 8 electrons in their valence shell (with the exception of helium which does not has only two electrons in its valence shell).
Thus, to achieve stability, oxygen contributes to one of its only electron pair in the formation of a coordinated covalent bond.
RELATED: What does the expression Time is a flat circle” mean in 2023
This results in the byte of oxygen (which was already present, because oxygen did not donate its electrons, it has just formed a coordinate bond and in a coordinate bond, a pair of electrons is shared between two atoms. but it is provided by a single atom). Carbon has now also reached its byte because previously it had six electrons, but now it has eight full electrons.
How there is no dative bond in CO3^2-?
For the formation of a dative bond, there should be at least a single pair of electrons in one of the atoms which are covalently bonded to the other atom and there should be a third atom which should have a deficit of at least a single pair of electrons
First of all, all ions such as SO4 (2-), NO3 (-), SO3 (2-), CO3 (2-), PO4 (3-), etc. are called acid radicals. In such radicals, there are no two oxygen atoms bonded together and all oxygen atoms are bonded to the central atom.
So in the case of Carbonate ion, we must first consider a covalent double bond between a carbon atom and an oxygen atom because a covalent bond is required for a coordinate bond.
No such molecule exists having a coordinated bond and not having a covalent bond. So after the formation of this covalent bond, carbon remains with a single pair of electrons and oxygen with three isolated pairs of electrons, as shown.
Carbon if it donates its only pair of electrons to form a coordinate bond, the third oxygen atom cannot be bonded to carbon and it will not form carbonate because carbonate has three oxygen atoms.
Oxygen cannot donate its only pair of electrons as this would cause a bond between two oxygen atoms and the carbonate radical could not be formed, so that there is no coordinated bond in the formation of the radical carbonate.
The real structure of the carbonate ion is like this, it has two oxygen atoms already having one excess electron and a third neutral oxygen atom.
RELATED: What does 0010110 mean in spirituality 2023?
Why CO is a lewis base?
Carbon monoxide is considered a Lewis base because it is well known to react with transition metals to form metal carbonyls.
In those reactions , It donates a pair of electrons to the metal cation (which is precisely what a Lewis base does!).
Below is a figure showing Back bonding in metal carbonyls :

How do carbon and oxygen have one lone pair each after forming a CO bond?
This is because after forming a double bond with oxygen, carbon has two electrons that do not participate in the bond and are always as one pair.
So carbon also has a vacant orbital. On the other hand, oxygen has two isolated pairs but no vacant orbitals.
The oxygen thus transfers its only pair to the vacant carbon orbital forming a dative bond. Thus, carbon and oxygen have a single pair each, even after the formation of CO.
What is the Lewis dot structure of CO?
The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure, you’ll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule.
RELATED: What does Return Service Requested Mean in USPS?
Is co linear or bent?
Carbon Monoxide is a diatomic molecule having ten valence electrons. Carbon and Oxygen atoms form triple bonds to complete their octets. Both these atoms have one lone pair of electrons and sp hybridization. Carbon Monoxide has a linear molecular geometry.
PEOPLE ALSO ASK FOR:
Lewis Structure Of CO2
Lewis Structure Of CH2O
Lewis Structure Of CO With Formal Charges
Lewis Structure Of CO32-
Write The Lewis Structure Of CO2
CO Lewis Structure Molecular Geometry
CO Structure
Consider The Lewis Structure Of CO .